Disease Diagnosis and Prevention Using CRISPR and Synthetic Biology
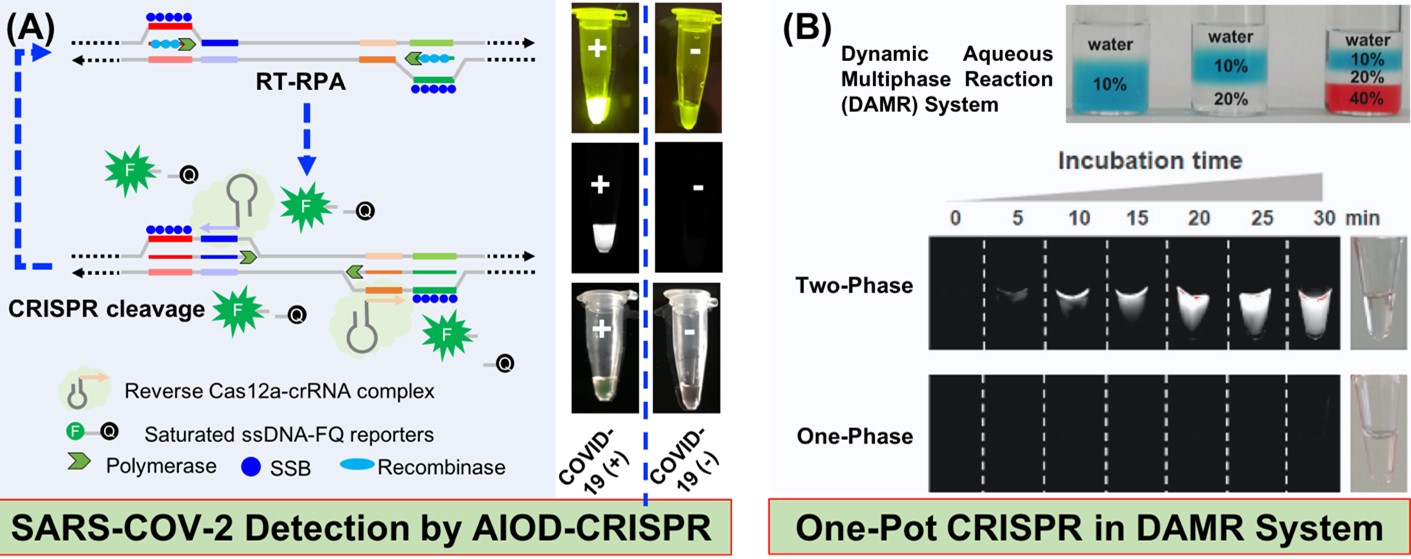
CRISPR (clustered regularly interspaced short palindromic repeats) technology and synthetic biology are revolutionizing the treatment, prevention and diagnosis of diseases. We are interested in the application of CRISPR technology and synthetic biology to disease diagnostics and treatment, in particular the use of CRISPR-toolbox/ synthetic biology to develop simple, rapid and sensitive diagnostic technologies. We have developed an All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) technology for rapid, ultrasensitive and visual detection of SARS-CoV-2 detection (Fig. A). We have proposed to a dynamic aqueous multiphase reaction (DAMR) system for one-pot CRISPR-Cas12a based nucleic acid quantitative detection (Fig. B).
Microfluidic Technology for Point of Care Molecular Diagnostics
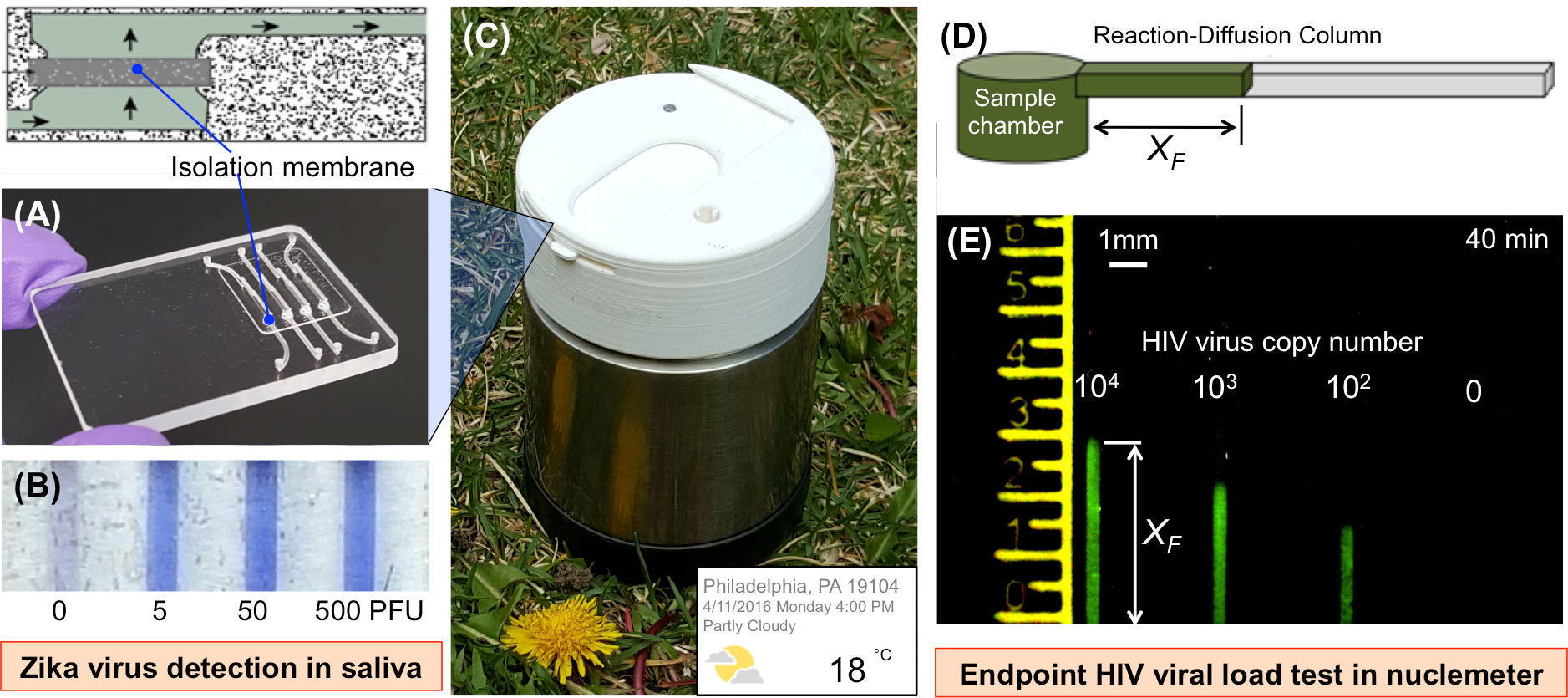
Rapid and timely molecular diagnosis of disease, including infectious disease and cancer, is critical for prompt and appropriate therapeutic intervention. However, current nucleic acid-based molecular diagnostics is still limited to laboratory use and not available for point of care (POC) diagnostic applications due to its requirement of expensive instrument and highly trained personnel. We have invented a number of novel POC diagnostic devices (Fig. A-E) for detecting HIV virus in saliva and plasma, zika virus (ZIKV) in saliva and urine, Cell-free circulating Schistosome mansoni DNA in serum, E. coli in urine and stool, and other diagnostic applications. We are employing multidisciplinary approaches to develop next-generation, low-cost, POC, molecular diagnostic devices for global health care and for individualized or customized medicine. For example, we are developing a new, reaction-diffusion-based, microfluidic method (dubbed “nuclemeter”) for quantifying nucleic acid molecules (Fig. D). The amount of target nucleic acid in raw clinical samples can be quantitatively read out through the position of polymerization reaction-diffusion front (XF) in the nuclemeter at the endpoint, enabling molecular diagnostics as simply as reading temperature in a “mercury in glass” thermometer (Fig. D and E).
Non-Instrumented Smartphone-based Diagnostic System
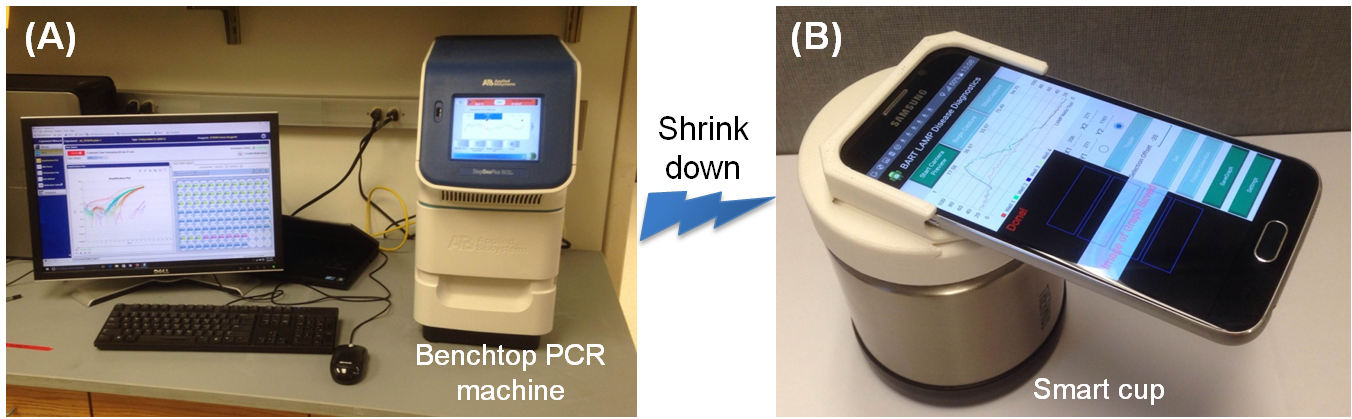
In many health care settings (at home, at clinics, et al), it is uneconomical, impractical, or unaffordable to maintain a well-equipped diagnostics laboratory. There is an urgent need for disposable diagnostic systems that require no complex instrument support (Fig. A), or significant training. Especially, the combination of inexpensive POC diagnostic device with ubiquitous smartphone-based detection technology will create a new paradigm shift for cost-effective, mobile, personalized, health monitoring in both the developed and the developing countries. We have exploited an inexpensive, electricity-free, thermal battery for nucleic acid amplification testing by taking advantage of exothermic reaction. We are leveraging ubiquitous smartphone technology to develop a non-instrumented, inexpensive, smartphone-based molecular diagnostic platform (dubbed the “smart cup”) (Fig. B), which may pave the way for the development of home-based molecular diagnostics (i.e., HIV viral load test at home, cervical cancer self screening) and mobile health (mHealth).
Centrifuge-free, Plasma/Serum Separation Method and Devices
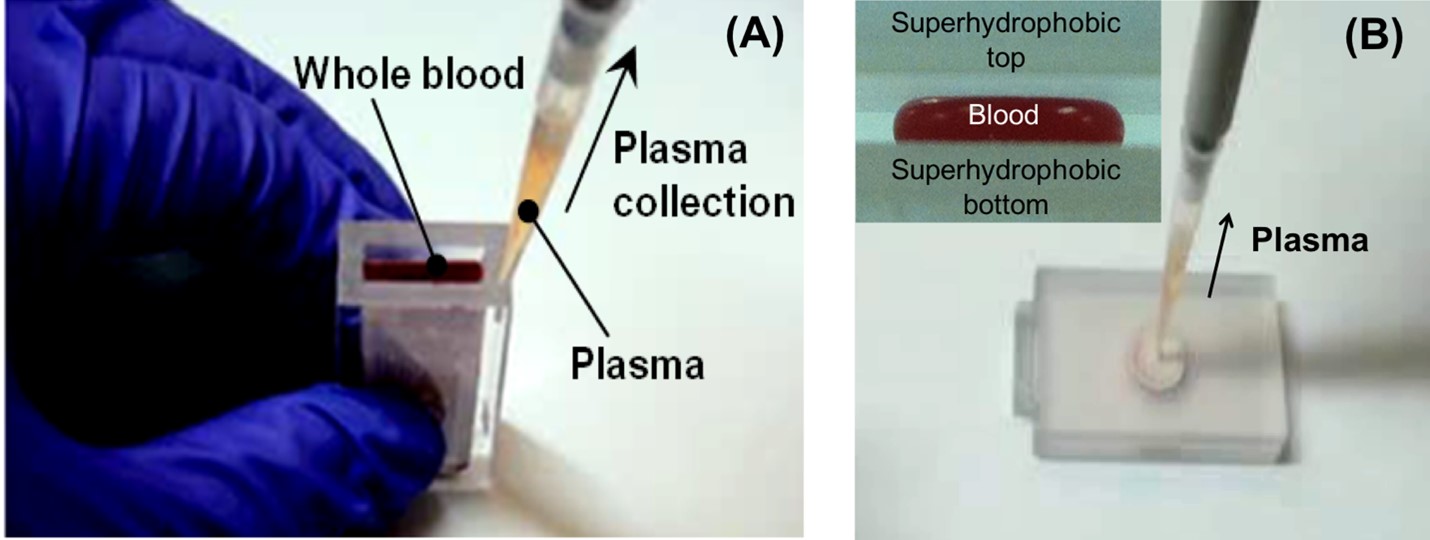
Plasma separation from raw whole blood is usually required for blood-based clinical diagnostics. Conventional centrifugation method is not suitable for on-site or bedside applications. To this end, we have developed and demonstrated various centrifuge-free plasma/serum separators based on different separation mechanisms (e.g., sedimentation-assisted (Fig. A), size exclusion-based filtration (Fig. B)). Our superhydrophobic plasma separator (Fig. B) shows a high plasma yield of above 70% with finger-prick blood (see video). The plasma yield is defined as the ratio of the volume of plasma extracted by the separator (Vseparator) and the volume of plasma separated by benchtop centrifugation (Vcentrifugation)). This technology has been licensed to companies for commercialization. We are further integrating our plasma separator with other diagnostic device to develop a fully integrated “blood-to-result” diagnostic system.